Anti-Human F10 & F9 activated form Recombinant Antibody
-
产品编号
YR1383
-
别名
科研级 Emicizumab ( 依米赛珠单抗 ), 艾米雪单抗,Anti-F10 & F9 activated form Recombinant Antibody, Research Grade Emicizumab
-
规格
- 1mg
- 5mg
| Catalog Number | YR1383 |
| Alias | 科研级 Emicizumab ( 依米赛珠单抗 ), 艾米雪单抗,Anti-F10 & F9 activated form Recombinant Antibody, Research Grade Emicizumab |
| Size | 1mg, 5mg |
| Molecular Name | Emicizumab |
| Product Introduction | 一种人源化双特异性单克隆抗体,以模拟凝血因子Ⅷ“桥接”功能的形式,将活化的凝血因子Ⅸ和凝血因子Ⅹ聚合以促进凝血。 |
| CAS Number | 1610943-06-0 |
| Target | F10 & F9 activated form[Homo sapiens] |
| Antibody Isotype | IgG4 Kappa |
| Clonity | Monoclonal |
| Concentration | 1mg/ml |
| Purity | >95% as determined by SDS-PAGE |
| SDS-PAGE | 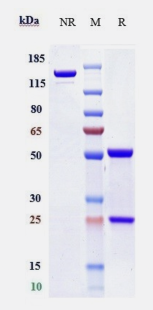 |
| Storage Condition | It is recommended to aliquote and store at -20°C for long term storage. Avoid repeated freezing and thawing cycles. |
| Formulation | PBS, pH7.5 |
| Shipping Condition | Shipped on ice packs. |
| Background | Emicizumab (trade name Hemlibra) is a humanized bispecific antibody for the treatment of haemophilia A, developed by Genentech and Chugai (a subsidiary of Roche). A Phase I clinical trial found that it was well tolerated by healthy subjects. |
| Remarks | This product is for research use only. |



 0
0
